Inspection of radiation sterilization
Writer: Sterilization Time: 2017-09-07 Browse: 849 ℃
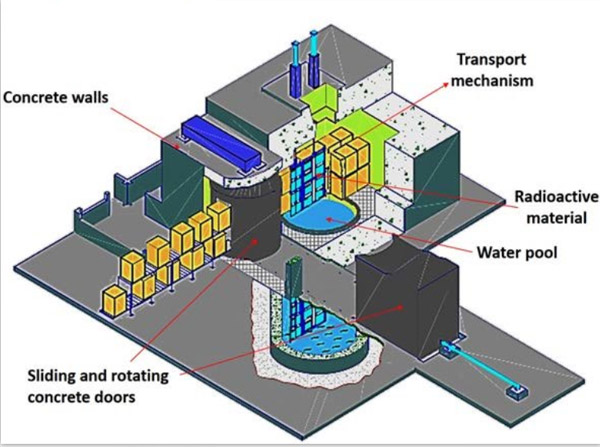
Inspection of radiation sterilization of cobalt 60
Due to the current national regulations on the use of radioactive elements, most of the domestic manufacturers using radiation sterilization take the form of commission to carry out sterilization activities. Therefore, the inspection of such sterilization activities should focus on whether the manufacturer understands the working principle of irradiation sterilization and the ability to monitor the sterilization process parameters of the entrusted party.
Verification of radiation sterilization
The radiation sterilization confirmation of the production enterprise can be completed together with the radiation sterilization unit, but the sterilization process control department of the production enterprise must participate in the whole process of the confirmation process, and can clearly describe the confirmation process.
1. Sterilization validation should include several components:
-
(1) Installation qualification of irradiation device: the qualification documents shall at least include the technical specifications and parameters of the irradiation device, the description of the irradiation device (source) location, the structure and parameters related to the transmission system, the size, material and structure of the irradiation container, the description of the operation of the irradiation device and the transmission system, and the certificate indicating the measurement date of the source activity, Verification certificate of time measuring equipment;
-
(2) Conduct product operation qualification in the irradiation device that has been installed and qualified: at least the dose distribution test report of products with uniform material shall be included in the operation qualification document, which shall indicate the relationship between irradiation time and absorbed dose of the product and the dose inhomogeneity;
-
(3) In the qualified equipment, the designated product or simulated product shall be used for performance appraisal: at least the dose distribution test report of the product shall be provided in the performance qualification document, and the report shall indicate the maximum dose / minimum dose value, area and non-uniformity, and shall be confirmed to meet the requirements by comparing with the maximum tolerance dose and minimum sterilization dose specified by the product. Before that, the influence of sterilization process on other properties of the product (such as chemical properties, physical properties, biocompatibility, etc.) should be confirmed to determine the maximum tolerable dose of the product.
-
(4) Establishment of sterilization dose: the sterilization dose shall be established in accordance with gb18280, and the report on the establishment of sterilization dose test shall at least include: initial pollution detection test report, recovery rate report of initial pollution test, verification dose test report, sterility test report and validation test report of sterility test;
-
(5) Establish the maximum tolerable dose: according to GB / t19633, the sterilization process validation experiment of packaging materials was completed to determine the maximum absorbed dose of packaging materials;
-
(6) If it is necessary to re sterilize, the re sterilization shall be confirmed, including inactivation confirmation and other performance confirmation (such as chemical properties, physical properties, biocompatibility, etc.);
-
(7) Review and approve documented quality management procedures;
-
(8) Support activities carried out to maintain validation.
2. Selection of products and packaging materials
The manufacturer shall consider the effects of irradiation on products (or product components) and packaging materials, and shall prove that the quality, safety and performance of products after radiation sterilization are guaranteed within the complete life cycle. Enterprises should consider: process, limit, radiation dose, radiation source, performance of raw materials and changes of storage environment when designing experimental procedures.
3. Radiation dose selection
The manufacturer should know the quantity and radiation resistance of natural microbial community on or in the product to determine the sterilization dose. The dose should be able to reach the pre selected sterility assurance level (SAL). When choosing the sterilization dose, we can use the bioburden information or the information obtained from the incremental dose experiment, or choose 25kgy or 15kgy as the sterilization dose after proving its suitability. The manufacturer should also regularly monitor the number of natural microbial populations present in the product to determine the effectiveness of the selected sterilization dose.
4. Determination of product loading mode
The production enterprise should establish its product loading mode for each product type. The technical specifications for this loading mode shall be documented as follows:
-
(1) Description of the packaged product, including size, density and acceptable deviation in this parameter, as well as the orientation of the product within the package when required;
-
(2) Description of the loading mode of the product in the irradiation container;
-
(3) Description of irradiation container machine dimensions.